doi: 10.56294/ri202469
ORIGINAL
Motor behavior improvement in ischemic gerbils by cholinergic receptor activation and treadmill training
Mejora del comportamiento motor en jerbos isquémicos mediante la activación de receptores colinérgicos y entrenamiento en cinta rodante
Lucas Hipolito do
Espírito Santo1 ![]() *, Kelly Zhang1
*, Kelly Zhang1
![]() *, Takae Tamy
Kitabatake1
*, Takae Tamy
Kitabatake1 ![]() *, Manoela Gallon
Pitta1
*, Manoela Gallon
Pitta1 ![]() *, Gustavo
Henrique de Mello Rosa1
*, Gustavo
Henrique de Mello Rosa1 ![]() *, Elaine
Caldeira de Oliveira Guirro1
*, Elaine
Caldeira de Oliveira Guirro1 ![]() *, João Eduardo
de Araujo1
*, João Eduardo
de Araujo1 ![]() *
*
1Ribeirão Preto Medical School University of São Paulo. Department of Health Sciences. Ribeirão Preto, Brazil.
Cite as: do Espírito Santo LH, Zhang K, Kitabatake TT, Gallon Pitta M, de Mello Rosa GH, de Oliveira Guirro EC, de Araujo joao eduardo. Motor behavior improvement in ischemic gerbils by cholinergic receptor activation and treadmill training. Interdisciplinary Rehabilitation / Rehabilitacion Interdisciplinaria 2024; 4:69. https://doi.org/10.56294/ri202469
Submitted: 16-11-2023 Revised: 22-01-2024 Accepted: 31-03-2024 Published: 01-04-2024
Editor:
Prof. Dr. Carlos Oscar Lepez ![]()
ABSTRACT
Introduction: treadmill exercise training is one of the most investigated non-pharmacological treatment options for experimental brain ischemia. However, the cholinergic system is essential for improving motor behavior responses.
Objective: to analyze the effects of a nicotinic acetylcholine receptor (nAChR) agonist (1, 2, and 4 mg/kg) on the motor behavior of ischemic gerbils subjected to forced treadmill training.
Methods: in this experimental study, 72 gerbils, weighing 65–80 g, were divided into eight groups: Sal, Ni1, Ni2, Ni4, I, INi1, INi2, and INi4. Behavioral assessment was initiated 24 hours after the last motor stimulation on the treadmill. Rotarod test (RR) was employed to analyze animal behavior. The data were analyzed using one-way analysis of variance (ANOVA), and the Newman-Keuls post hoc test evidenced differences detected between groups.
Results: data regarding the RR test revealed decreased time spent on the RR apparatus for the Ni1, Ni4, and I group compared to the Sal and Ni2 groups. However, the INi1 and INi2 groups showed increased time spent compared with the ischemia and INi4 groups (F7,64=4,63; p<0,05).
Conclusions: the present study indicates that treadmill training with a concomitant 1 and 2 mg/kg of nAChR agonist effectively improves the behavior of ischemic gerbils.
Keywords: Gerbillinae; Brain Ischemia; Cholinergic Agonists; Rotarod Performance Test; Motor Activity.
RESUMEN
Introducción: el entrenamiento con cinta rodante es una de las opciones de tratamiento no farmacológico más investigadas para la isquemia cerebral experimental. Sin embargo, el sistema colinérgico es esencial para mejorar las respuestas del comportamiento motor.
Objetivo: analizar los efectos de un agonista del receptor de acetilcolina nicotínica (nAChR) (1, 2 y 4 mg/kg) en el comportamiento motor de jerbos isquémicos sometidos a un entrenamiento forzado en cinta rodante.
Métodos: en este estudio experimental, 72 jerbos, con un peso de 65-80 g, se dividieron en ocho grupos: Sal, Ni1, Ni2, Ni4, I, INi1, INi2 y INi4. La evaluación del comportamiento se inició 24 horas después de la última estimulación motora en la cinta rodante. Se empleó la prueba del Rotarod (RR) para analizar el comportamiento animal. Los datos se analizaron mediante análisis de varianza unidireccional (ANOVA), y la prueba post hoc de Newman-Keuls evidenció diferencias detectadas entre los grupos.
Resultados: los datos sobre la prueba RR revelaron un tiempo disminuido en el aparato RR para los grupos Ni1, Ni4 e I en comparación con los grupos Sal y Ni2. Sin embargo, los grupos INi1 e INi2 mostraron un tiempo aumentado en comparación con los grupos de isquemia e INi4 (F7,64=4,63; p<0,05).
Conclusiones: el presente estudio indica que el entrenamiento en cinta rodante con un agonista de nAChR de 1 y 2 mg/kg mejora eficazmente el comportamiento de jerbos isquémicos.
Palabras clave: Gerbillinae; Isquemia Cerebral; Agonistas Colinérgicos; Prueba de Rendimiento Rotarod; Actividad Motriz.
INTRODUCTION
Ischemic stroke occurs when the blood supply to the brain is interrupted, leading to reduced local oxygenation and damage to brain tissue.(1) This condition accounts for approximately 80% of all stroke cases.(2) To better understand and advance the rehabilitation tools for stroke patients, experimental animal models of brain ischemia are crucial due to inherent limitations in human studies.(3,4)
Due to the absence of a complete brain Willis polygon, Gerbils are particularly susceptible to global ischemic injury, with no communication between the middle and posterior cerebral arteries.(5) It is important to note that ischemia with reperfusion in gerbil models results in distinct structural and behavioral changes compared to ischemia without reperfusion.(6,7) Using a bilateral carotid occlusion model for 5 minutes followed by reperfusion results in significant damages to the striatum, hippocampal CA1 area, and motor cortex M.(7)
Previous studies have shown that treadmill training can potentially prevent neuronal death, particularly in the hippocampus, which is responsible for memory, and other areas associated with motor behavior.(7,8,9) The results of previous studies underscore the importance of optimizing the training dose.(10)
Given the ongoing discussion surrounding time window, target, and treatment duration in animal stroke and drug therapy models,(11) this study explores the potential of concomitant drug therapy with exercise. The literature suggests that activating nicotinic cholinergic receptors (nAChR) plays a pivotal role in motor behavior by recruiting motor pathways in both naive and treated rats.(12) These receptors act as ligand-gated cation channels in the cholinergic system, impacting learning, memory, autonomic response, and motor control.(13)
In light of the evidence suggesting that nAChR agonists and treadmill training can enhance motor behavior, this study aims to analyze the effects of a nicotinic acetylcholine receptor (nAChR) agonist (1, 2, and 4 mg/kg) on the motor behavior of ischemic gerbils subjected to forced treadmill training. The hypothesis is that the concomitant treatment with a nAChR agonist will potentiate the effects of treadmill training. Furthermore, the dose-effect curves at 1, 2, and 4 mg/kg will be explored.
MATERIALS AND METHODS
Animals
This experimental study was carried out in the Laboratory of Neuropsychobiology and Motor Behavior of the Ribeirão Preto Medical School of the University of São Paulo (FMRP-USP). The animals of this study, weighing between 65-80 g, were divided into eight groups, involving a total of seventy-two gerbils (Meriones unguiculatus; Rodentia Gerbillinae). The gerbils remained in the laboratory at a controlled temperature (23±1°C) under lighting conditions (12/12 h) in polypropylene cages (30 × 20 × 13 cm), with free access to water and food during the entire period of the experiment.
Surgery
The animals were placed on the surgical table under intraperitoneal anesthesia with Zoletil® (60 mg/kg), receiving a ventral incision in the neck. Then, their subcutaneous and muscle tissues were pushed back to expose and bilaterally occlude the external carotid artery for 5 minutes using a silk suture. The area was irrigated with sterile sodium chloride to prevent tissue dehydration. In addition, a visual check was performed to ensure that the blood flow was interrupted. After 5 minutes, the arteries were released, allowing reperfusion in the area, followed by suturing the incision using sterile silk thread (Shalon-3.0). After the surgical procedure, the animals received pentabiotic (120,000 IU) to prevent infection and were placed on a hot plate until they regained consciousness to avoid hypothermia.
Drug Application
The nAChR agonist nicotine sulfate (pyrrolidine, 1 methyl-2-(3-pyridyl)-, sulfate; grade II; PM 422-6; Sigma®, Brazil) was used. The drug was administered for nine consecutive days during the experiment: three days before the experimental surgery, on the day of surgery, and five days after the forced treadmill training. Each animal received an intraperitoneal injection of 1, 2, or 4 mg/kg of the nAChR agonist in the drug group. The drug was dissolved in saline, adjusting the volume so that 1 mg of the nAChR agonist was present in 2 ml of saline.
The data showed that the drug affected balance, motor planning, and animal coordination during this period.(15) Therefore, to prevent drug action during surgery or motor stimulation on a treadmill, the application was performed 30 min after these procedures.
Experimental Design
All animals were assigned to one of the following groups after they underwent treadmill training (n=9):
· Sal - Animals subjected to experimental surgery without occlusion of the carotid artery (interrupted before occlusion of the carotid) and injected with saline intraperitoneal injection.
· Ni1, Ni2, and Ni4 (nAChR agonist doses of 1 mg/kg, 2 mg/kg, and 4 mg/kg, respectively) - Animals subjected to experimental surgery without occlusion of the carotid artery (interrupted before occlusion of the carotid) and injected with respective doses of nAChR agonist intraperitoneal injection.
· I - Animals subjected to experimental surgery for carotid occlusion and administered an intraperitoneal injection of saline solution.
· INi1, INi2, and INi4 (Ischemia with nAChR agonist doses of 1 mg/kg, 2 mg/kg, and 4 mg/kg, respectively) - Animals subjected to experimental surgery for carotid occlusion and injected with nAChR agonist intraperitoneal injection.
Treadmill training
For the training were used a motorized treadmill for rodents (Insight Ltd., São José dos Campos, Brazil) consisting of six boxes, each measuring 40 inches in length and 10 inches in width. To ensure adherence to the required speed, the treadmill is equipped with an electrical stimulation device on its back that encourages the animal to maintain the established pace. The stimulation protocol was set at a fixed speed of 5 m/min for 15 min, with a 0 % grade of inclination, without speed increases and a total stimulation period of five days.(8)
Motor behavior test
All animals were habituated in Rotarod (Insight Ltd., São José dos Campos, Brazil) for three consecutive days before the experimental surgery. All animals returned to the RR test on the sixth day after experimental surgery (see the timeline in Figure 1). This equipment includes a cylindrical surface that rotates around its axis at an acceleration of 5.5 rpm. Animals were individually placed inside the moving cylinder, and the test ended when the animal fell off the cylinder. The trial was repeated three times and recorded. The data obtained were equivalent to the average of three sessions in the mobile cylinder.(7,16)
When the animals completed the test, they were weighed and received terminal anesthesia using an excessive dose of Zoletil (180 mg/kg) and then exsanguinated by cardiac puncture.

RR: Rotarod.
Figure 1. Experiment timeline
Statistical analysis
The statistical tests were conducted using the SigmaPlot®13 program. The RR test data were initially analyzed using a Shapiro-Wilk test for normality assessment, followed by a one-way analysis of variance (ANOVA). Subsequently, the Newman-Keuls post hoc test was used to identify differences between the groups. Statistical significance was considered when the P-value was less than 0,05.
Ethical aspects
All recommendations of the Committee on Ethics in Animal Experimentation of FMRP-USP (Proc. 077/2014) from the Brazilian College of Animal Ethics in Research were followed. Furthermore, to reduce animal suffering and minimize the number of animals used, the experimental design recommendations reported by the ARRIVE guidelines for animals used in research were adopted.(14) Also, this manuscript adheres to the principles of the 3Rs (Replacement, Reduction, and Refinement). The study design prioritizes the ethical use of animals in research, seeking alternatives where possible (Replacement), minimizing the number of animals used (Reduction), and refining experimental procedures to enhance animal welfare (Refinement). By embracing the 3Rs, this research contributes to the ongoing commitment to responsible and humane treatment of laboratory animals.(17)
RESULTS
The RR showed a decreased time spent in the test in the Ni1, Ni4, and ischemia groups compared to the Sal and Ni2 groups. However, the INi1 and INi2 groups showed an increase in the time spent compared to the ischemia and INi4 groups (F7,64=4,63; p<0,05) (Figure 2).
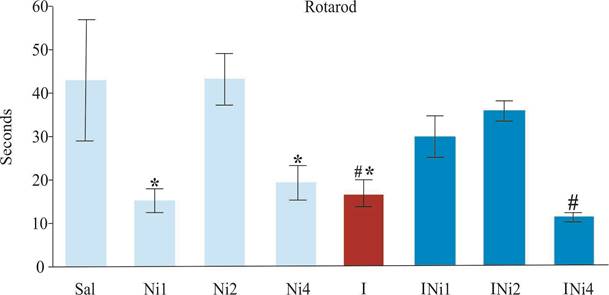
The data are reported as the mean ± Standard Error of Mean. *Statistically significant decrease compared to Sal and Ni2; # Statistically significant decrease compared to INi1 and INi2, according to the one-way ANOVA, followed by Newman-Keuls post hoc, p<0,05. Number of animals in all groups = 9.
Figure 2. Time spent on the Rotarod (RR) test
DISCUSSION
The present study investigated the effects of three doses of the nAChR agonist and its capacity to improve the well-known effects of treadmill physical exercise training in ischemic gerbils.(8,9,16) In addition, several studies have shown that physical exercise can increase neuronal growth and decrease neuronal death after brain ischemia.(18,19,20) Furthermore, it is already well-established in the literature that using this nAChR agonist for nine days does not generate neuroprotective effects.(20) In this study, the nAChR agonist associated with treadmill training provided new evidence that the cholinergic system potentiates motor behavior in sham and ischemic gerbils.
The point of discussion regarding treadmill training is the dose-related differences between several protocols.(22) Additionally, studies of early initiated training and low-to-moderate intensity showed the protection of the behavior and morphological impairment mitigation imposed by brain ischemia.(23) Thus, in this study, the behavioral improvement of ischemic gerbils in the RR test can be attributed to low-intensity treadmill training, as shown in other studies.(8,15) Furthermore, the RR test is a standard test that can measure motor behavior and the activity of brain structures such as the striatum and hippocampal CA1 area, which are essential for good performance in the test.(7) Since the understanding of neuronal protection following treadmill training is already well-established, this aspect will not be explored.
The focus of the current investigation was to highlight the impact of nAChR agonists on treadmill training and to investigate the effects of three nAChR agonist doses, which yielded different results. The 1 and 2 mg/kg doses potentiated the treadmill training effects in ischemic animals. However, this effect in sham animals is distinct, as only the 2 mg/kg dose had a favorable effect, which is in line with another study that used the same dose in untrained rats,(23) nAChR agonists have a stimulant effect that increases motor behavioral activity.(24) In addition, nAChR agonists inhibit monoamine oxidase (MAO), which is responsible for the breakdown of dopamine. With an increase in circulating dopamine levels, there is an increase in the positive reinforcing effects of nAChR agonists.(25)
Furthermore, by binding to the nAChR agonist acetylcholine receptors, the nAChR agonist influences learning, memory, and cognition.(26) Activation of these receptors by nAChR agonists can increase or directly induce long-term hippocampal potentiation, a form of synaptic plasticity related to long-term memory formation.(24) It is also well established that its administration regulates neurotrophins for neuronal maintenance during an ischemic event.(27) In this sense, the hypothesis is that the nAChR agonist at 1 and 2 mg/kg in ischemic gerbils reinforces the learning effects of previous training in the RR and the protective effect of exercise.
Interestingly, 4 mg/kg of the nAChR agonist produced the opposite effect in the ischemic animals. In humans, the plasma levels of nAChR agonists in moderate smokers are between 10 and 100 ng/ml. In contrast, chronic smokers have > 100 ng/ml.(28) Based on this classification, doses between 0,5 to 5 mg/kg reach plasma concentrations corresponding to moderate levels in human smokers.(28) Interestingly, this high dose had the opposite effect and negatively affected the behavior of sham animals. Reduced animal activity is observed when large drug doses are administered shortly before a brief test session.(29,30) After training, a high nAChR agonist dose reversed the training effects, and the animals required more trials to provide the correct response. This behavioral impairment is related to the striatum's low brain-derived neuronal growth factor (BDNF)(31) These two points support our evidence to explain the shorter time the ischemic and sham animals spent on high nAChR agonist doses in the RR test. With the RR test's previous learning reversed and lower BDNF levels in the striatum, the ischemic animal could not provide good motor behavior during the test. Treadmill-trained animals in the sham group did not have impairments in the striatum, but the high dose did affect previous learning.
The study should be interpreted with a focus on its assessment capabilities. The limitations of this behavioral study are acknowledged, taking into account previous research that demonstrated morphological changes following ischemia and treadmill training.(8,16)
CONCLUSIONS
The present study shows that treadmill training with a concomitant 1 and 2 mg/kg of the nAChR agonist effectively improves behavior in sham and ischemic gerbils and points to a dose-response curve with an excessive 4 mg/kg nAChR agonist dose. However, further studies are needed to understand the influence of nAChR agonists on ischemia-exercised gerbils. Therefore, exercise pharmacology rehabilitation may be proposed to reverse post-ischemic motor impairment in the future.
REFERENCES
1. Kuriakose D, Xiao Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int J Mol Sci 2020;21(20):7609. doi: 10.3390/ijms21207609
2. Truelsen T, Bonita R, Jamrozik K. Surveillance of stroke: a global perspective. Int J Epidemiol 2001;30. doi: 10.1093/ije/30.suppl_1.s11
3. Lee JC, Won MH. Neuroprotection of antioxidant enzymes against transient global cerebral ischemia in gerbils. Anat Cell Biol 2014;47(3):149-156. doi: 10.5115/acb.2014.47.3.149
4. Wahl AS, Schwab ME. Finding an optimal rehabilitation paradigm after stroke: enhancing fiber growth and training of the brain at the right moment. Front Hum Neurosci 2014;8:381. doi: 10.3389/fnhum.2014.00381.
5. Du XY, Zhu XD, Dong G, et al. Characteristics of circle of Willis variations in the mongolian gerbil and a newly established ischemia-prone gerbil group. ILAR Journal. 2011 ;52(1):E1-7. doi: 10.1093/ilar.52.1.e1.
6. Lipton P. Ischemic cell death in brain neurons. Physiol Rev 1999;79(4):1431-1568. doi: 10.1152/physrev.1999.79.4.1431
7. de Araujo FL, Bertolino G, Gonçalves RB, et al. Neuropathology and behavioral impairments after three types of global ischemia surgery in Meriones unguiculatus: evidence in motor cortex, hippocampal CA1 region and the neostriatum. J Neurol Sci 2012;312(1-2):73-78. doi: 10.1016/j.jns.2011.08.019
8. Kitabatake TT, Marini LC, Gonçalves RB, et al. Behavioral effects and neural changes induced by continuous and not continuous treadmill training, post bilateral cerebral ischemia in gerbils. Behav Brain Res 2015;291:20-25. doi: 10.1016/j.bbr.2015.04.057
9. Silveira APC, Kitabatake TT, Pantaleo VM, Zangrossi H Júnior, Bertolino G, de Oliveira Guirro EC, de Souza HCD, de Araujo JE. Continuous and not continuous 2-week treadmill training enhances the performance in the passive avoidance test in ischemic gerbils. Neurosci Lett 2018;665:170-175. doi: 10.1016/j.neulet.2017.12.012
10. Austin MW, Ploughman M, Glynn L, Corbett D. Aerobic exercise effects on neuroprotection and brain repair following stroke: a systematic review and perspective. Neurosci Res 2014;87:8-15. doi: 10.1016/j.neures.2014.06.007
11. Kaur H, Prakash A, Medhi B. Drug Therapy in Stroke: From Preclinical to Clinical Studies. Pharmacology 2013;92(5-6):324-334. doi: 10.1159/000356320
12. Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 2005;15(2):161-167. doi: 10.1016/j.conb.2005.03.004
13. Lipovsek M, Fierro A, Pérez EG, et al. Tracking the Molecular Evolution of Calcium Permeability in a Nicotinic Acetylcholine Receptor, Molecular Biology and Evolution, 2014;31(12):3250–3265. https://doi.org/10.1093/molbev/msu258
14. Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18(7):e3000410. doi: 10.1371/journal.pbio.3000410
15. Welch KD, Pfister JA, Lima FG, Green BT, Gardner DR. Effect of α₇ nicotinic acetylcholine receptor agonists and antagonists on motor function in mice. Toxicol Appl Pharmacol 2013;266(3):366-374. doi: 10.1016/j.taap.2012.11.024
16. Buiatti de Araujo FL, Bertolino G, Rodrigues Funayama CA, et al. Influence of treadmill training on motor performance and organization of exploratory behavior in Meriones unguiculatus with unilateral ischemic stroke: histological correlates in hippocampal CA1 region and the neostriatum. Neurosci Lett. 2008;431(2):179-83. doi: 10.1016/j.neulet.2007.11.038.
17. Hubrecht RC, Carter E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals. 2019; 9(10):754. https://doi.org/10.3390/ani9100754
18. Zhao Y, Pang Q, Liu M, Pan J, Xiang B, Huang T, Tu F, Liu C, Chen X. Treadmill Exercise Promotes Neurogenesis in Ischemic Rat Brains via Caveolin-1/VEGF Signaling Pathways. Neurochem Res 2017;42(2):389-397. doi: 10.1007/s11064-016-2081-z
19. Luo L, Li C, Du X, Shi Q, Huang Q, Xu X, Wang Q. Effect of aerobic exercise on BDNF/proBDNF expression in the ischemic hippocampus and depression recovery of rats after stroke. Behav Brain Res 2019;362:323-331. doi: 10.1016/j.bbr.2018.11.037
20. Zhang D, Lu Y, Zhao X, Zhang Q, Li L. Aerobic exercise attenuates neurodegeneration and promotes functional recovery - Why it matters for neurorehabilitation & neural repair. Neurochem Int 2020;141:104862. doi: 10.1016/j.neuint.2020.104862
21. Seyedaghamiri F, Mahmoudi J, Hosseini L, Sadigh-Eteghad S, Farhoudi M. Possible Engagement of Nicotinic Acetylcholine Receptors in Pathophysiology of Brain Ischemia-Induced Cognitive Impairment. J Mol Neurosci 2022;72(3):642-652. doi: 10.1007/s12031-021-01917-4
22. Wogensen E, Malá H, Mogensen J. The Effects of Exercise on Cognitive Recovery after Acquired Brain Injury in Animal Models: A Systematic Review. Neural Plast 2015;2015:830871. doi: 10.1155/2015/830871
23. Royal W 3rd, Can A, Gould TD, Guo M, Huse J, Jackson M, Davis H, Bryant J. Cigarette smoke and nicotine effects on brain proinflammatory responses and behavioral and motor function in HIV-1 transgenic rats. J Neurovirol 2018;24(2):246-253. doi: 10.1007/s13365-018-0623-7
24. Kutlu MG, Gould TJ. Nicotine modulation of fear memories and anxiety: Implications for learning and anxiety disorders. Biochem Pharmacol 2015;97(4):498-511. doi: 10.1016/j.bcp.2015.07.029
25. Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Shea C, Schlyer D, Wolf AP, Warner D, Zezulkova I, Cilento R. Inhibition of monoamine oxidase B in the brains of smokers. Nature 1996;379(6567):733-736. doi: 10.1038/379733a0
26. Riljak V, Maresova D, Pokorny J. Nicotine effects on rat seizures susceptibility and hippocampal neuronal degeneration. Neuro Endocrinol Lett 2010;31(6):792-795. PMID: 21196917
27. Jackson KJ, Muldoon PP, De Biasi M, Damaj MI. New mechanisms and perspectives in nicotine withdrawal. Neuropharmacology 2015;96(Pt B):223-234. doi: 10.1016/j.neuropharm.2014.11.009
28. Benowitz NL, Jacob P 3rd, Herrera B. Nicotine intake and dose response when smoking reduced-nicotine content cigarettes. Clin Pharmacol Ther 2006;80(6):703-714. doi: 10.1016/j.clpt.2006.09.007
29. Bradford ST, Stamatovic SM, Dondeti RS, Keep RF, Andjelkovic AV. Nicotine aggravates the brain postischemic inflammatory response. Am J Physiol Heart Circ Physiol 2011;300(4):H1518-H1529. doi: 10.1152/ajpheart.00928.2010
30. Clarke PB, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Br J Pharmacol 1983;78(2):329-337. doi: 10.1111/j.1476-5381.1983.tb09398.x
31. Ortega LA, Tracy BA, Gould TJ, Parikh V. Effects of chronic low- and high-dose nicotine on cognitive flexibility in C57BL/6J mice. Behav Brain Res 2013;238:134-145. doi: 10.1016/j.bbr.2012.10.032
FINANCING
This work was funded by the São Paulo Research Foundation (FAPESP)
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest to declare.
ACKNOWLEDGMENTS
This work was funded by the São Paulo Research Foundation (FAPESP -2005/02607-3). Lucas H. E. Santo, Kelly Zhang, and Manoela G. Pitta received scientific initiation fellowship funding from FAPESP (2019/10320–9, 2019/06286–0, and 2019/07817–9), respectively. Takae T. Kitabatake received master fellowship funding from FAPESP (2015/07730-0).
AUTHORS' CONTRIBUTION
Conceptualization: João E. de Araujo.
Formal analysis: Gustavo H. de Mello Rosa, Elaine C. O. Guirro.
Funding acquisition: João E. de Araujo.
Investigation: Lucas H. E. Santo, Kelly Zhang, Manoela G. Pitta.
Methodology: Takae Tamy Kitabatake
Writing - original draft: Lucas H. do Espírito Santo, Kelly Zhang
Writing - review & editing: João E. de Araujo.